in the production process of biologics
Amino acids in the production process of biologics.
Amino acids are important biomolecules as they are the building blocks of proteins. In the production process of biologics, they play multifaceted crucial roles as they can be used as bulking agents, stabilizers, excipients, isotonicity and viscosity adjusters, or antioxidants.
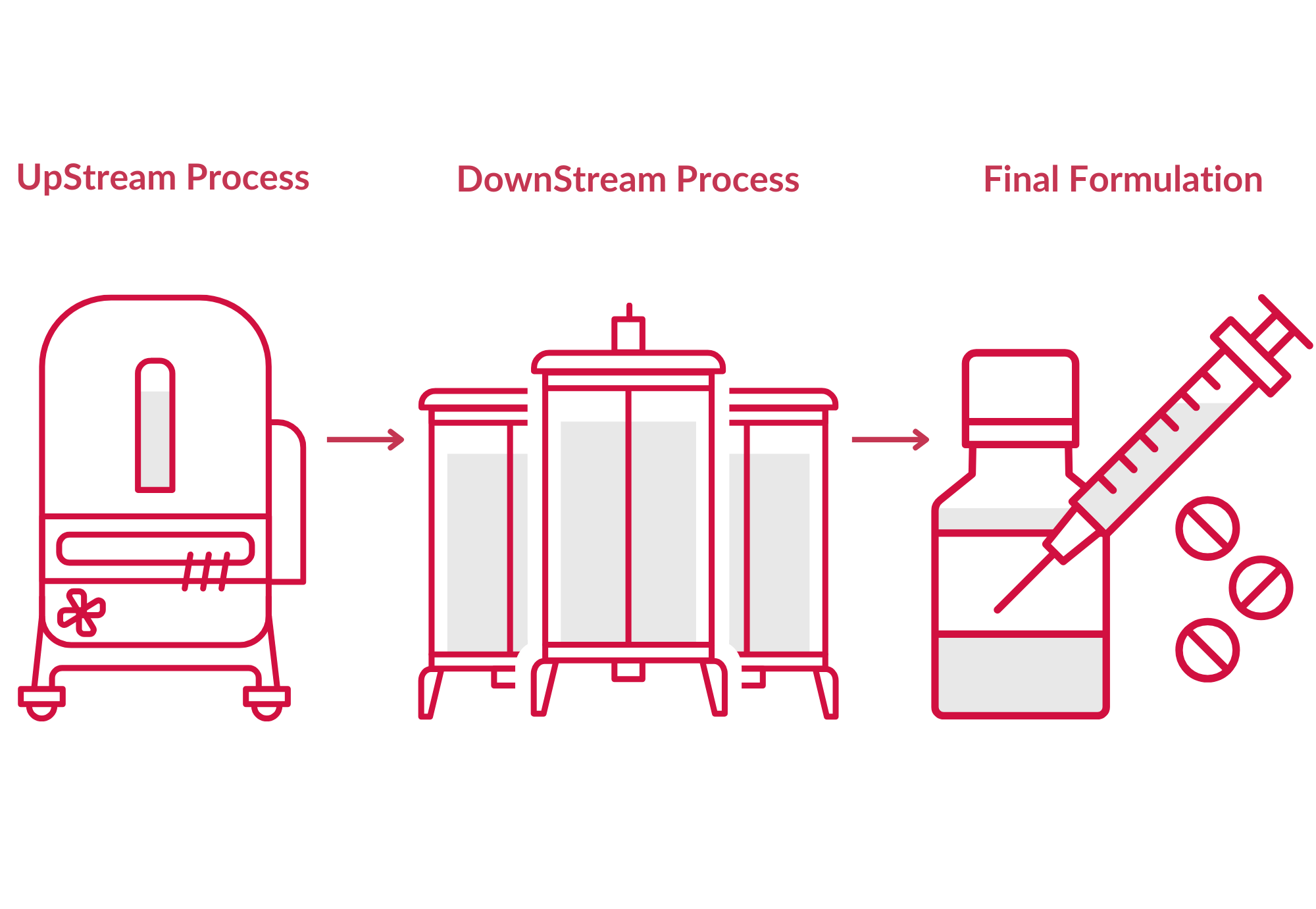
Upstream process (USP)
USP covers every stage of the microorganism's development that takes place in the fermentation process: nutrient preparation, cell culture, cell separation, and harvesting. Aim: to attain large-scale cell development from small quantities using a range of cell lines.
During USP, amino acids are included in the culture media as they are the main source of nitrogen. They are therefore crucial for the proliferation of cells and allow to determine the maximum cell density thanks to their concentration. L-glutamine is the most used supplement in cell culture.
DownStream Process (DSP)
DSP consists of isolating, purifying, and concentrating the previously produced biological drug substance.
Amino acids can serve during the DSP extraction and purification stages as:
-
stabilizers (glycine, Histidine and Arginine)
-
antioxidants (methionine and Cysteine)
-
buffering agents (histidine, glycine and glutamic acid)
Some practical examples :
-
Affinity chromatography: introducing hydrophilic amino acids to fusion tag enhances the solubility of the molecule of interest.[1]
-
Protein refolding via dilution: amino acids, such as glycine, can be included in the composition of refolding buffers to increase the success of refolding. Moreover, arginine, combined with other molecules, was reported to suppress precipitation during refolding and increase solubility. [2]
-
Cleavage of fusion proteins: lysine and arginine linkers can serve as trypsin cleavage site to cleave fusion proteins.[3]
-
Reverse-phase chromatography: protonation of the carboxylic acid groups on the side chains of glutamic and aspartic acids will make the protein more hydrophobic at a low pH. [4][5]
Final formulation (FF)
FF is a procedure that converts a biological drug substance into a formulated drug product thanks to the addition of excipients such as minerals, emulsifiers, polyols but also amino acids.
There exist two possible final forms for the biological drug products: liquid and solid (usually powder). Liquid dosage formulations often have a lower shelf life than their solid counterparts due to their inherent instability.
A solution to this problem is the use of lyophilization. The conversion of the liquid formulation into a solid form will significantly extend product stability and thus its shelf life. Products that have been lyophilized can be easily reconstituted by diluting the powder with an appropriate solvent, like sterile water.
The most commonly used amino acids in FF are: glycine, arginine, histidine, leucine, proline and lysine. Glycine provides bulk and structure to the lyophilized cake, arginine is a great anti-aggregation protein agent while histidine and proline can be used as stabilizing agents.
For a biologics production process, the amino acids must at least have a pharma grade and low endotoxin measure if used in the final formulation.
Get in touch
Contact us for more information and support in your biologics development process!
 e-mail us
e-mail us

